Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
How can we convert CO₂ from threat to asset?
Chemists want to use electrochemistry to turn the greenhouse gas into chemical feedstocks and fuels, but they need to improve their systems’ efficiencies first
by Leigh Krietsch Boerner
October 11, 2020
| A version of this story appeared in
Volume 98, Issue 39
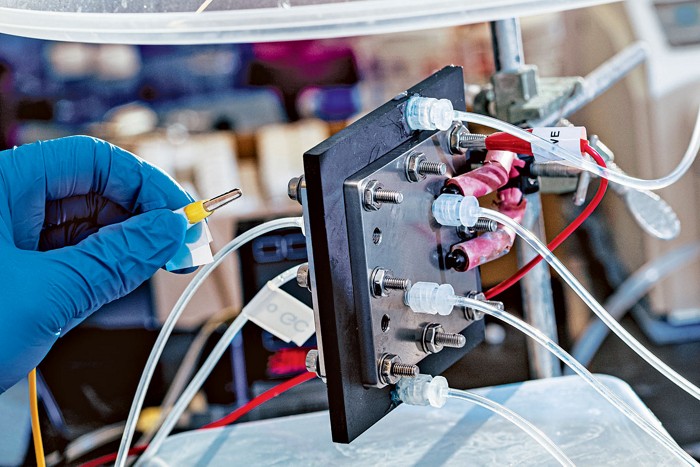
It’s no secret that humans have emitted too much carbon dioxide. Now catastrophic climatic changes caused by this gaseous glut are unrolling before our eyes. While some scientists and engineers are working on ways to tamp down CO2 emissions, others want to figure out what to do with the gas we’ve already released into the atmosphere.
Some want to snag and sequester CO2, usually by pumping it deep into the earth. Others propose growing plants to grab the gas and then using the resulting biomass to make useful things, such as chemicals and plastics. One option that scientists have recently fallen hard for is called carbon capture and utilization. After taking CO2 from the air, chemists would reduce the gas and turn it into fuels and raw ingredients for chemical manufacturing. So far in 2020, scientists have published over 1,700 papers on the subject. In 2010, there were just under 500 publications.
From a cost perspective, the most promising way to turn CO2 into useful chemicals is electrochemistry. Electrochemical cells move electrons through a system to drive a reaction, in this case the reduction of CO2. The approach faces one major hurdle: low efficiency.
Right now, the overall efficiency of systems that reduce CO2 electrochemically hovers around 20–22%. This figure is a measurement of how well the system converts the incoming electricity into the desired product. So a cell running at 20% overall efficiency means that 80% of the energy chemists put in doesn’t go toward making what they want. Such low efficiency is a giant waste, says Ted Sargent, an electrical engineer at the University of Toronto who has worked on CO2 reduction for more than 5 years.
According to economic models, for this approach to be a practical route to chemicals and fuels, chemists would need to improve its efficiency to around 50 or 60%. Below that efficiency, the amount of electricity needed to drive these reactions would cost more than the value of the chemicals produced.
So chemists are looking at the different components of these cells for ways to increase efficiency. Some are trying to make more specific catalysts that reduce the energy input needed to get the reaction going. Others think there needs to be more research into the electrolytes and electrodes used in these cells.
“To compete, at least purely on cost and in an unsubsidized world, we’re going to have to improve energy efficiency,” Sargent says. But, researchers say, the how is not so easy.
Electrochemical selling points
Electrochemistry offers several advantages over other chemical approaches to CO2 reduction, such as driving the reactions thermally. First, chemists can use renewable energy sources like wind and solar to provide the electricity to run the reactions instead of burning fossil fuels to heat them.
Scientists could use electricity to make heat to drive thermal chemistry, but this strategy presents a couple of problems, Sargent says. One is that thermal methods to reduce CO2 make a large mix of carbon-containing products. Researchers can separate these products to get the ones they want, but that process comes at a price, both in energy use and complexity of the process. As a result, costs go up, he says.
Also, using heat to drive industrial reactions requires large plant designs because the processes have to run in large reactors under extreme conditions to make the syntheses cost effective. Large plants are expensive to build. Electrochemical routes offer a way to scale down chemical plants, Sargent says.

“These methods can actually operate under room temperature and ambient pressure,” says Haotian Wang, an electrochemist at Rice University. So industrial chemists could design smaller plants in many areas instead of one big plant in a single area. A capital investment of billions of dollars to build a large CO2-reduction plant could be a big barrier for a new business getting started, Sargent says. As a result, it’s likely more feasible, cost-wise and size-wise, for companies to get into carbon capture and utilization through electrochemical systems.
Electrochemical reduction of CO2 could also help solve one of the problems with renewable electricity sources. These energy sources produce electricity intermittently. “If everybody goes home and does their laundry at six o’clock, unfortunately, the wind doesn’t pick up automatically at 6:00 p.m.,” Sargent says. “You need to move energy around in time.” Batteries can store a few hours’ worth of energy. But for longer-term storage—say, moving solar energy collected in the summer into energy to heat our homes in the winter—you need something with greater energy-storing capacity. A fuel like methane or ethanol could do the trick, Sargent says. We already know how to move fuels around, he says; the infrastructure exists.
And the best way to convert CO2 to a fuel is through electrochemistry, according to Sargent. “It is the most literal and direct route,” he says.
The sum of its parts
Electrochemical systems that convert CO2 into useful products like fuels have a lot of parts that need to work together. A membrane splits the cell in two, with an anode in one half and a cathode in the other. An electrolyte fills the cell, allowing current to flow from the anode to the cathode.
US chemical costs
Electrochemical CO2-reducing systems need to reach 50–60% efficiency to be cost effective. Below that figure, the amount of electricity needed to drive the reactions would cost more than the value of the chemicals produced, which can vary greatly.
Methanea: $0.00008 per liter
Methanol: $0.24 per liter
Ethanea: $0.0002 per liter
Ethanol: $0.38 per liter
Ethylenea: $0.0004 per liter
Propane: $0.22 per liter
Sources: US Energy Information Administration, Methanex 2020 annual report, Westlake Chemical 2019 annual report, US Grains Council. Note: Prices for ethane, ethylene, and propane are from 2019, the year of the most recent report. All other prices are from 2020. a Compound is normally sold as a gas and will appear price deflated because it has a low mass per unit volume.
CO2 comes into the system and interacts with the cathode. A catalyst embedded in or on the cathode takes electrons flowing through the electrode and reduces the CO2 to the desired product. The membrane allows selective diffusion of hydroxide from the cathode side of the cell to the anode side, where the other half of the electrochemical reaction happens, usually oxidation of water to oxygen. Sounds simple, right? But the timing of the events matters.
“CO2 gets reduced at an electrode, and you need protons and electrons both interacting with CO2 to convert it into something,” says Curtis Berlinguette, a materials chemist from the University of British Columbia. Generally, the protons come from water, so CO2, water, and electrons all have to meet up at exactly the right time, without one building up into an excess, Berlinguette says. “Otherwise you start creating problems.” For example, with an excess of one reactant, the reduction may stall, the catalyst may get poisoned and stop working, or an unwanted side reaction might take over.
When these cells are working together, they can have high specificity. And that can be a bit of a disadvantage. If scientists want to change the chemical they are making, they need to build an entirely new system with a different catalyst, cathode, anode, and electrolyte. A setup that makes ethanol, for example, cannot suddenly start making methane instead.
If such electrochemical systems do get efficient enough to work on large scales, deciding which products to make will depend largely on economics. The market price for the carbon-based products is more complicated than the compound’s size. Even though methane is a fuel that can store a lot of energy, the market value is low, Sargent says. Ethanol is better, and the values of other C2 compounds can be higher still, depending on energy storage capacity and potential uses by the chemical sector. C3, C4, and C5 compounds are worth more, but the price starts to drop as the carbon chains get longer. “Then you actually start to lose value per kilowatt-hour of energy,” Sargent says. Most researchers are focusing on making C1 and C2 compounds before moving on to larger and more valuable compounds.
Improving the catalyst
While scientists have made great improvements in the efficiencies of these CO2-reducing cells in the past 5 years or so, Sargent says, they still have a ways to go yet. Improving the catalysts is probably the most crowded area of research. The catalyst is critical: it controls how much energy is needed to run the reaction and determines what product gets made.
One source of catalyst inefficiency is a factor called the overpotential. It is the difference between how much energy is theoretically needed to drive a reaction and how much energy a reaction actually requires.

For example, say you want to split water into oxygen and hydrogen. The reaction needs 1.2 electron volts (eV). “That means the minimum energy you need to put in there for a reaction is 1.2 eV,” says Xile Hu, an energy chemist from the Swiss Federal Institute of Technology, Lausanne (EPFL). “In reality, you probably need 2 eV,” he says. The amount of energy between the two numbers, 0.8 eV, is the overpotential.
The overpotential comes from losses in the system, energy that doesn’t go where researchers want it to go—that is, toward making products. If you can run the reaction with closer to 1.2 instead of 2 eV, then the system is not wasting as much energy, and its efficiency increases, Hu says.
Tinkering with a catalyst can shrink the overpotential, Hu says. Just like a catalyst in a thermal reaction, an electrocatalyst can stabilize reactants so that a step in the reaction path requires less energy. One issue is that CO2 reduction reactions have many steps, says Samira Siahrostami, a computational chemist at the University of Calgary. She models how catalysts reduce CO2 to CO and then on to methane. With a carbon catalyst she studies, the reaction requires eight protons and electrons, she says. “These protons and electrons will be added to CO2, each one a single step. So that means this reaction requires eight different elementary steps,” she says. The step with the highest overpotential determines the overpotenial for the whole system.
Her group uses computational methods to calculate the reaction pathway that requires the least amount of energy associated with different catalysts. Then, to find ways to reduce their energy needs, the researchers look at the steps from that route that cost the most energy. Working with Rice’s Wang, Siahrostami and coworkers have used these methods to determine that the efficiency difference between nickel- and cobalt-embedded graphene nanosheet catalysts in reducing CO2 to CO was due to distinct reaction pathways.
The overall efficiency depends not only on the overpotential but also on a system’s Faradaic efficiency. This value is a measure of how selective the electrocatalyst is. How much of the flow of electrons in a system goes toward making the desired product instead of side products?
Currently, larger products lead to lower Faradaic efficiencies. For example, making CO and formic acid involves only a two-electron transfer. “These two are the simplest products of CO2 reduction, so that’s why you can deliver higher selectivity and higher production rates,” Wang says. As the compounds get more complicated, the selectivity drops. “What happens is that your electrolyzer starts spewing out a range of different products,” the University of British Columbia’s Berlinguette says. So more and more electrons start going toward reactions that make side products instead of toward the intended one.
As a result, a lot of researchers have focused their efforts on catalysts that reduce CO2 to make CO. The reaction is simpler, more selective, and attractive to industry because it’s already in use, Hu says. The chemical industry uses a mixture of CO and H2 called syngas, short for synthesis gas, to make other chemicals, such as ammonia or methanol. It’s also used as a precursor to lubricants and fuels. With CO plus H2, researchers can make nearly everything industry makes from petroleum. “If you can make renewable carbon monoxide, you have a big place in the chemical industry,” Hu says.
Despite CO2-to-CO catalysts’ high selectivity, there is room for improvement. One approach to increase selectivity is to simplify the catalyst. Hu and coworkers made CO with 90% Faradaic efficiency with an Fe(III) single-atom catalyst. For chemists, working with single-atom catalysts makes it easier to understand how the catalyst is behaving during the reaction, allowing them to track “who is doing what,” Hu says. However, single-atom catalysts generally have a slow reaction rate. But in Hu’s catalyst, the researchers embedded the iron atom in a carbon framework and doped it with nitrogen. The nitrogen coordinates with the Fe and stabilizes it, Hu says.
Advertisement
Through calculations, the team found that its Fe(III) catalyst is highly efficient because the metal species always stays in the Fe(III) oxidation state. “This is completely counterintuitive,” Hu says. “When you have a reduction, you reduce your metal, right?” But in this system, the Fe doesn’t get reduced, because the carbon-and-nitrogen framework stabilizes the metal, which allows Fe to merge electronically with the carbon support. “And so, basically every time you pass the electron to the Fe, it doesn’t take it; it just passes it to the carbon support,” Hu says. Compared with other Fe oxidation states, Fe(III) is particularly fast at absorbing CO2 and has a low affinity for CO. That means it can grab CO2 quickly, reduce it to CO, and then release the product before any side reactions can occur. This quality improves selectivity, leading to a higher Faradaic efficiency.
Another way to boost Faradaic efficiency is to play Le Chatelier’s game of forcing the reaction equilibrium toward the desired products. Hongjie Dai and coworkers at Stanford University increased their system’s Faradaic efficiency to 98%by using high pressures of CO2. The system involved a copper oxide nanoparticle catalyst that reduces CO2 to formate. The team used 4.56 MPa (45 atm) of CO2 to increase the amount of CO2 dissolved in the electrolyte. More CO2 in the electrolyte helped tilt the equilibrium more strongly toward making formate over other products. The system’s overall efficiency was 55%. However, using high pressures could make it hard for chemists to scale up the system because of safety concerns, Dai says.
Beyond the catalyst
While most groups have focused on improving the catalyst’s efficiency, other parts of these electrochemical cells need help too.
Few people work on electrolytes for the CO2-reduction system, says Alissa Park, a chemical engineer from Columbia University. An efficient electrolyte moves chemical species around in the cell to get them where they need to go, to either the cathode or the anode. Many electrolytes are water based, which is the general electrochemical go-to. But CO2 is not very soluble in water, Park says. In a cell that contains an electrolyte with a high water concentration, the amount of CO2 available for the cathode catalyst may not be sufficient to keep reaction rates up.
Park’s group has designed one alternative electrolyte that excels at pulling in CO2. The materials, called nanoparticle organic hybrid materials (NOHMs), are “basically a nanoparticle tethered with polymer chains,” she says. The tethered polymer chains make the materials liquid-like, allowing them to serve as an electrolyte in an electrochemical cell. Functional groups on these polymeric arms, such as amines or ethers, grab onto CO2 through weak electrostatic interactions. When the CO2 held in the NOHMs’ arms gets reduced to CO, it pops off because the functional groups can’t hold CO as efficiently as CO2.
Although these materials can provide more CO2 for reduction at cathodes than water-based electrolytes can, they probably won’t find immediate use. Getting enough CO2 to the electrode isn’t the biggest limit on a system’s efficiency right now, because the catalysts aren’t converting the CO2 to product fast enough, Park says. “But one day, once we have better catalysts, you will be limited by supply of CO2,” she says. Also, the NOHMs are too sticky to flow, so Park’s group is working on adding a secondary liquid to lower their viscosity.
Experts in the field agree that the anodes in these cells also need optimization. But most researchers are focused on other components and haven’t spent time to figure out how exactly to improve the electrodes.
Despite the challenges with all these cells’ components, Sargent says, scientists have made progress on efficiency rates. “We were definitely below 10% energy efficiency just a couple of years ago,” he says. The research community has mobilized and worked hard on the problems. That creates a positive feedback loop, he says. “And I don’t think we’re done yet.”
Correction
This story was updated on Oct. 19, 2020, to correct the chemical costs table. The price for methane is $0.00008 per liter, not $0.08. Also the price for propane was originally labeled as the price for propanol. The table also now denotes which compounds are normally sold as gases.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter