Reports
Sale

Global In-Vitro Diagnostics Market Size, Share, Forecast: By Product and Service: Reagents and Kits, Instruments, Software and Services; By Technology: Immunodiagnostics, Clinical Chemistry, Molecular Diagnostics, Haematology, Others; By Application; By End Use; Regional Analysis; Market Dynamics; Competitive Landscape; 2024-2032
Global In-Vitro Diagnostics Market Outlook
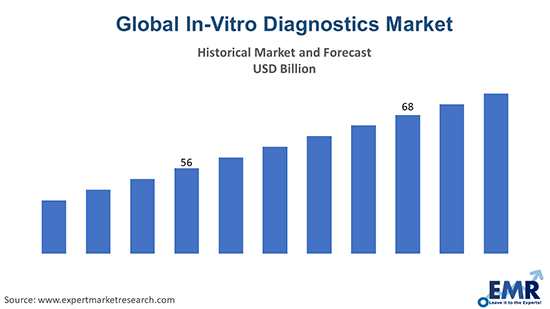
The global in-vitro diagnostics market reached a value of about USD 71.90 billion in 2023. The market is further expected to grow at a CAGR of 4.5% in the forecast period of 2024-2032 to reach a value of approximately USD 106.79 billion by 2032.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Increasing Demand for Point of Care Testing to Augment the In-Vitro Diagnostics Industry Growth
Based on end-use, the point of care (POC) testing segment accounts for a significant share in the in-vitro diagnostics industry. This can be attributed to the development of technology that has made POC testing seamless and quick, thus, making it the first choice of customers. Further, they shorten a 5 step traditional approach to a simple 2 step process, which makes the testing quick as well as cost-effective. The rising demand for convenience is significantly contributing to the growth of the segment.
The Asia Pacific to Provide Enhanced Growth Opportunities to the In-Vitro Diagnostics Industry
Region-wise, the Asia Pacific is expected to witness a steady growth over the forecast period owing to the increasing demand for advanced diagnostic facilities, improving healthcare infrastructure, and rising standard of living. Within Asia Pacific, China is one of the key markets for the product. Furthermore, medical inspection revenue has also increased in the region, which, in turn, is leading to an increased demand for IVD products.
In-Vitro Diagnostics: Market Segmentation
In-vitro diagnostics (IVDs) refer to the medical tests performed on blood or tissue samples that have been taken from the human body. In-vitro diagnostics can detect diseases such as HIV and cholera and can be used to keep a check on a patient’s overall health to help cure, treat, or prevent diseases. In-vitro diagnostics are used to identify specific treatments that can give added benefits to patients over other treatments. Furthermore, with advancements in the field of in vitro diagnostics, there has been a growing focus on next-generation sequencing (NGS) tests, which can scan a person’s DNA to detect genomic variations.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
By product and service, the market is divided into:
- Reagents and Kits
- Instruments
- Software and Services
Based on technology, the industry can be segmented into:
- Immunodiagnostics
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Enzyme-Linked Immunospot (ELISPOT)
- Rapid Tests
- Radioimmunoassay (RIA)
- Western Blotting
- Others
- Clinical Chemistry
- Basic Metabolic Panels
- Liver Panels
- Renal Profiles
- Lipid Profiles
- Thyroid Function Panels
- Electrolyte Panels
- Specialty Chemical Tests
- Others
- Molecular Diagnostics
- Polymerize Chain Reaction (PCR)
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Hybridisation
- DNA diagnostics
- Microarray
- Others
- Haematology
- Microbiology
- Coagulation and Haemostasis
- Urinalysis
- Others
The market is divided based on application into:
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Drug Testing/Pharmacogenomics
- HIV/Aids
- Autoimmune Diseases
- Nephrology
- Others
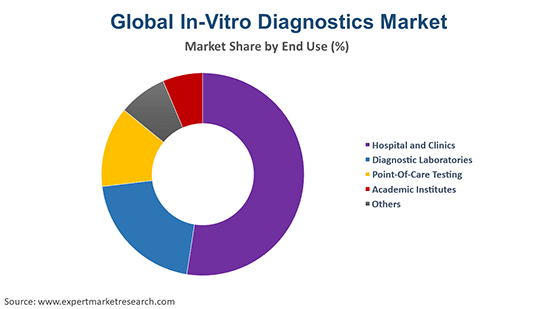
On the basis of end use, the industry can be categorised into:
- Hospital and Clinics
- Diagnostic Laboratories
- Point-Of-Care Testing
- Academic Institutes
- Others
The regional markets for the product include North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Increasing Demand for Rapid Testing and the Rise in Prevalence of Chronic Diseases to Bolster the Growth of the In-Vitro Diagnostics Industry
In recent years, in-vitro diagnostics has witnessed a surge in demand owing to the incresaing demand for advanced diagnostic facilities and a dramatic shift towards rapid testing. There has been a rise in the adoption of in-vitro diagnostics like point of care testing, PCR testing, and DNA diagnostics. During times of COVID-19, rapid testing has emerged as the primary method of identifying infected people. Furthermore, the rise in the prevalence of chronic as well as lifestyle disorders is creating a huge market for the in-vitro diagnostics industry.
Over the forecast period, the rapid technological advancements and the increasing focus on safety and accuracy are expected to favour the industry growth. The rise in chronic diseases is expected to further propel the industry forward.
Key Industry Players in the Global In-Vitro Diagnostics Market
The report gives a detailed analysis of the following key players in the global in-vitro diagnostics market, covering their competitive landscape, capacity, and latest developments like mergers, acquisitions, and investments, expansions of capacity, and plant turnarounds:
- Danaher Corp
- bioMérieux, Inc
- Siemens Healthcare GmbH
- ARKRAY America, Inc.
- Sysmex Corporation
- F. Hoffmann-La Roche Ltd
- Others
The comprehensive EMR report provides an in-depth assessment of the market based on the Porter's five forces model along with giving a SWOT analysis.
Key Highlights of the Report
| REPORT FEATURES | DETAILS |
| Base Year | 2023 |
| Historical Period | 2017-2023 |
| Forecast Period | 2024-2032 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Product and Service |
|
| Breakup by Technology |
|
| Breakup by Application |
|
| Breakup by End Use |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Competitive Landscape |
|
| Companies Covered |
|
| Report Price and Purchase Option | Explore our purchase options that are best suited to your resources and industry needs. |
| Delivery Format | Delivered as an attached PDF and Excel through email, with an option of receiving an editable PPT, according to the purchase option. |
*At Expert Market Research, we strive to always give you current and accurate information. The numbers depicted in the description are indicative and may differ from the actual numbers in the final EMR report.
1 Preface
2 Report Coverage – Key Segmentation and Scope
3 Report Description
3.1 Market Definition and Outlook
3.2 Properties and Applications
3.3 Market Analysis
3.4 Key Players
4 Key Assumptions
5 Executive Summary
5.1 Overview
5.2 Key Drivers
5.3 Key Developments
5.4 Competitive Structure
5.5 Key Industrial Trends
6 Snapshot
6.1 Global
6.2 Regional
7 Opportunities and Challenges in the Market
8 Global In-Vitro Diagnostics Market Analysis
8.1 Key Industry Highlights
8.2 Global In-Vitro Diagnostics Historical Market (2018-2023)
8.3 Global In-Vitro Diagnostics Market Forecast (2024-2032)
8.4 Global In-Vitro Diagnostics Market by Product and Service
8.4.1 Reagents and Kits
8.4.1.1 Historical Trend (2018-2023)
8.4.1.2 Forecast Trend (2024-2032)
8.4.2 Instruments
8.4.2.1 Historical Trend (2018-2023)
8.4.2.2 Forecast Trend (2024-2032)
8.4.3 Software and Services
8.4.3.1 Historical Trend (2018-2023)
8.4.3.2 Forecast Trend (2024-2032)
8.5 Global In-Vitro Diagnostics Market by Technology
8.5.1 Immunodiagnostics
8.5.1.1 Historical Trend (2018-2023)
8.5.1.2 Forecast Trend (2024-2032)
8.5.1.3 Breakup by Type
8.5.1.3.1 Enzyme-Linked Immunosorbent Assay (ELISA)
8.5.1.3.1.1 Historical Trend (2018-2023)
8.5.1.3.1.2 Forecast Trend (2024-2032)
8.5.1.3.2 Enzyme-Linked Immunospot (ELISPOT)
8.5.1.3.2.1 Historical Trend (2018-2023)
8.5.1.3.2.2 Forecast Trend (2024-2032)
8.5.1.3.3 Rapid Tests
8.5.1.3.3.1 Historical Trend (2018-2023)
8.5.1.3.3.2 Forecast Trend (2024-2032)
8.5.1.3.4 Radioimmunoassay (RIA)
8.5.1.3.4.1 Historical Trend (2018-2023)
8.5.1.3.4.2 Forecast Trend (2024-2032)
8.5.1.3.5 Western Blotting
8.5.1.3.5.1 Historical Trend (2018-2023)
8.5.1.3.5.2 Forecast Trend (2024-2032)
8.5.1.3.6 Others
8.5.2 Clinical Chemistry
8.5.2.1 Historical Trend (2018-2023)
8.5.2.2 Forecast Trend (2024-2032)
8.5.2.3 Breakup by Type
8.5.2.3.1 Basic Metabolic Panels
8.5.2.3.1.1 Historical Trend (2018-2023)
8.5.2.3.1.2 Forecast Trend (2024-2032)
8.5.2.3.2 Liver Panels
8.5.2.3.2.1 Historical Trend (2018-2023)
8.5.2.3.2.2 Forecast Trend (2024-2032)
8.5.2.3.3 Renal Profiles
8.5.2.3.3.1 Historical Trend (2018-2023)
8.5.2.3.3.2 Forecast Trend (2024-2032)
8.5.2.3.4 Lipid Profiles
8.5.2.3.4.1 Historical Trend (2018-2023)
8.5.2.3.4.2 Forecast Trend (2024-2032)
8.5.2.3.5 Thyroid Function Panels
8.5.2.3.5.1 Historical Trend (2018-2023)
8.5.2.3.5.2 Forecast Trend (2024-2032)
8.5.2.3.6 Electrolyte Panels
8.5.2.3.6.1 Historical Trend (2018-2023)
8.5.2.3.6.2 Forecast Trend (2024-2032)
8.5.2.3.7 Specialty Chemical Tests
8.5.2.3.7.1 Historical Trend (2018-2023)
8.5.2.3.7.2 Forecast Trend (2024-2032)
8.5.2.3.8 Others
8.5.3 Molecular Diagnostics
8.5.3.1 Historical Trend (2018-2023)
8.5.3.2 Forecast Trend (2024-2032)
8.5.3.3 Breakup by Type
8.5.3.3.1 Polymerize Chain Reaction (PCR)
8.5.3.3.1.1 Historical Trend (2018-2023)
8.5.3.3.1.2 Forecast Trend (2024-2032)
8.5.3.3.2 Isothermal Nucleic Acid Amplification Technology (INAAT)
8.5.3.3.2.1 Historical Trend (2018-2023)
8.5.3.3.2.2 Forecast Trend (2024-2032)
8.5.3.3.3 Hybridisation
8.5.3.3.3.1 Historical Trend (2018-2023)
8.5.3.3.3.2 Forecast Trend (2024-2032)
8.5.3.3.4 DNA diagnostics
8.5.3.3.4.1 Historical Trend (2018-2023)
8.5.3.3.4.2 Forecast Trend (2024-2032)
8.5.3.3.5 Microarray
8.5.3.3.5.1 Historical Trend (2018-2023)
8.5.3.3.5.2 Forecast Trend (2024-2032)
8.5.3.3.6 Others
8.5.4 Haematology
8.5.4.1 Historical Trend (2018-2023)
8.5.4.2 Forecast Trend (2024-2032)
8.5.5 Microbiology
8.5.5.1 Historical Trend (2018-2023)
8.5.5.2 Forecast Trend (2024-2032)
8.5.6 Coagulation and Haemostasis
8.5.6.1 Historical Trend (2018-2023)
8.5.6.2 Forecast Trend (2024-2032)
8.5.7 Urinalysis
8.5.7.1 Historical Trend (2018-2023)
8.5.7.2 Forecast Trend (2024-2032)
8.5.8 Others
8.6 Global In-Vitro Diagnostics Market by Application
8.6.1 Infectious Diseases
8.6.1.1 Historical Trend (2018-2023)
8.6.1.2 Forecast Trend (2024-2032)
8.6.2 Diabetes
8.6.2.1 Historical Trend (2018-2023)
8.6.2.2 Forecast Trend (2024-2032)
8.6.3 Oncology
8.6.3.1 Historical Trend (2018-2023)
8.6.3.2 Forecast Trend (2024-2032)
8.6.4 Cardiology
8.6.4.1 Historical Trend (2018-2023)
8.6.4.2 Forecast Trend (2024-2032)
8.6.5 Drug Testing/Pharmacogenomics
8.6.5.1 Historical Trend (2018-2023)
8.6.5.2 Forecast Trend (2024-2032)
8.6.6 HIV/Aids
8.6.6.1 Historical Trend (2018-2023)
8.6.6.2 Forecast Trend (2024-2032)
8.6.7 Autoimmune Diseases
8.6.7.1 Historical Trend (2018-2023)
8.6.7.2 Forecast Trend (2024-2032)
8.6.8 Nephrology
8.6.8.1 Historical Trend (2018-2023)
8.6.8.2 Forecast Trend (2024-2032)
8.6.9 Others
8.7 Global In-Vitro Diagnostics Market by End Use
8.7.1 Hospital and Clinics
8.7.1.1 Historical Trend (2018-2023)
8.7.1.2 Forecast Trend (2024-2032)
8.7.2 Diagnostic Laboratories
8.7.2.1 Historical Trend (2018-2023)
8.7.2.2 Forecast Trend (2024-2032)
8.7.3 Point-Of-Care Testing
8.7.3.1 Historical Trend (2018-2023)
8.7.3.2 Forecast Trend (2024-2032)
8.7.4 Academic Institutes
8.7.4.1 Historical Trend (2018-2023)
8.7.4.2 Forecast Trend (2024-2032)
8.7.5 Others
8.8 Global In-Vitro Diagnostics Market by Region
8.8.1 North America
8.8.1.1 Historical Trend (2018-2023)
8.8.1.2 Forecast Trend (2024-2032)
8.8.2 Europe
8.8.2.1 Historical Trend (2018-2023)
8.8.2.2 Forecast Trend (2024-2032)
8.8.3 Asia Pacific
8.8.3.1 Historical Trend (2018-2023)
8.8.3.2 Forecast Trend (2024-2032)
8.8.4 Latin America
8.8.4.1 Historical Trend (2018-2023)
8.8.4.2 Forecast Trend (2024-2032)
8.8.5 Middle East and Africa
8.8.5.1 Historical Trend (2018-2023)
8.8.5.2 Forecast Trend (2024-2032)
9 North America In-Vitro Diagnostics Market Analysis
9.1 United States of America
9.1.1 Historical Trend (2018-2023)
9.1.2 Forecast Trend (2024-2032)
9.2 Canada
9.2.1 Historical Trend (2018-2023)
9.2.2 Forecast Trend (2024-2032)
10 Europe In-Vitro Diagnostics Market Analysis
10.1 United Kingdom
10.1.1 Historical Trend (2018-2023)
10.1.2 Forecast Trend (2024-2032)
10.2 Germany
10.2.1 Historical Trend (2018-2023)
10.2.2 Forecast Trend (2024-2032)
10.3 France
10.3.1 Historical Trend (2018-2023)
10.3.2 Forecast Trend (2024-2032)
10.4 Italy
10.4.1 Historical Trend (2018-2023)
10.4.2 Forecast Trend (2024-2032)
10.5 Others
11 Asia Pacific In-Vitro Diagnostics Market Analysis
11.1 China
11.1.1 Historical Trend (2018-2023)
11.1.2 Forecast Trend (2024-2032)
11.2 Japan
11.2.1 Historical Trend (2018-2023)
11.2.2 Forecast Trend (2024-2032)
11.3 India
11.3.1 Historical Trend (2018-2023)
11.3.2 Forecast Trend (2024-2032)
11.4 ASEAN
11.4.1 Historical Trend (2018-2023)
11.4.2 Forecast Trend (2024-2032)
11.5 Australia
11.5.1 Historical Trend (2018-2023)
11.5.2 Forecast Trend (2024-2032)
11.6 Others
12 Latin America In-Vitro Diagnostics Market Analysis
12.1 Brazil
12.1.1 Historical Trend (2018-2023)
12.1.2 Forecast Trend (2024-2032)
12.2 Argentina
12.2.1 Historical Trend (2018-2023)
12.2.2 Forecast Trend (2024-2032)
12.3 Mexico
12.3.1 Historical Trend (2018-2023)
12.3.2 Forecast Trend (2024-2032)
12.4 Others
13 Middle East and Africa In-Vitro Diagnostics Market Analysis
13.1 Saudi Arabia
13.1.1 Historical Trend (2018-2023)
13.1.2 Forecast Trend (2024-2032)
13.2 United Arab Emirates
13.2.1 Historical Trend (2018-2023)
13.2.2 Forecast Trend (2024-2032)
13.3 Nigeria
13.3.1 Historical Trend (2018-2023)
13.3.2 Forecast Trend (2024-2032)
13.4 South Africa
13.4.1 Historical Trend (2018-2023)
13.4.2 Forecast Trend (2024-2032)
13.5 Others
14 Market Dynamics
14.1 SWOT Analysis
14.1.1 Strengths
14.1.2 Weaknesses
14.1.3 Opportunities
14.1.4 Threats
14.2 Porter’s Five Forces Analysis
14.2.1 Supplier’s Power
14.2.2 Buyer’s Power
14.2.3 Threat of New Entrants
14.2.4 Degree of Rivalry
14.2.5 Threat of Substitutes
14.3 Key Indicators for Demand
14.4 Key Indicators for Price
15 Value Chain Analysis
16 Competitive Landscape
16.1 Market Structure
16.2 Company Profiles
16.2.1 Danaher Corp
16.2.1.1 Company Overview
16.2.1.2 Product Portfolio
16.2.1.3 Demographic Reach and Achievements
16.2.1.4 Certifications
16.2.2 bioMérieux, Inc
16.2.2.1 Company Overview
16.2.2.2 Product Portfolio
16.2.2.3 Demographic Reach and Achievements
16.2.2.4 Certifications
16.2.3 Siemens Healthcare GmbH
16.2.3.1 Company Overview
16.2.3.2 Product Portfolio
16.2.3.3 Demographic Reach and Achievements
16.2.3.4 Certifications
16.2.4 ARKRAY America, Inc.
16.2.4.1 Company Overview
16.2.4.2 Product Portfolio
16.2.4.3 Demographic Reach and Achievements
16.2.4.4 Certifications
16.2.5 Sysmex Corporation
16.2.5.1 Company Overview
16.2.5.2 Product Portfolio
16.2.5.3 Demographic Reach and Achievements
16.2.5.4 Certifications
16.2.6 F. Hoffmann-La Roche Ltd
16.2.6.1 Company Overview
16.2.6.2 Product Portfolio
16.2.6.3 Demographic Reach and Achievements
16.2.6.4 Certifications
16.2.7 Others
17 Key Trends and Developments in the Market
List of Key Figures and Tables
1. Global In-Vitro Diagnostics Market: Key Industry Highlights, 2018 and 2032
2. Global In-Vitro Diagnostics Historical Market: Breakup by Product and Service (USD Billion), 2018-2023
3. Global In-Vitro Diagnostics Market Forecast: Breakup by Product and Service (USD Billion), 2024-2032
4. Global In-Vitro Diagnostics Historical Market: Breakup by Technology (USD Billion), 2018-2023
5. Global In-Vitro Diagnostics Market Forecast: Breakup by Technology (USD Billion), 2024-2032
6. Global In-Vitro Diagnostics Historical Market: Breakup by Application (USD Billion), 2018-2023
7. Global In-Vitro Diagnostics Market Forecast: Breakup by Application (USD Billion), 2024-2032
8. Global In-Vitro Diagnostics Historical Market: Breakup by End Use (USD Billion), 2018-2023
9. Global In-Vitro Diagnostics Market Forecast: Breakup by End Use (USD Billion), 2024-2032
10. Global In-Vitro Diagnostics Historical Market: Breakup by Region (USD Billion), 2018-2023
11. Global In-Vitro Diagnostics Market Forecast: Breakup by Region (USD Billion), 2024-2032
12. North America In-Vitro Diagnostics Historical Market: Breakup by Country (USD Billion), 2018-2023
13. North America In-Vitro Diagnostics Market Forecast: Breakup by Country (USD Billion), 2024-2032
14. Europe In-Vitro Diagnostics Historical Market: Breakup by Country (USD Billion), 2018-2023
15. Europe In-Vitro Diagnostics Market Forecast: Breakup by Country (USD Billion), 2024-2032
16. Asia Pacific In-Vitro Diagnostics Historical Market: Breakup by Country (USD Billion), 2018-2023
17. Asia Pacific In-Vitro Diagnostics Market Forecast: Breakup by Country (USD Billion), 2024-2032
18. Latin America In-Vitro Diagnostics Historical Market: Breakup by Country (USD Billion), 2018-2023
19. Latin America In-Vitro Diagnostics Market Forecast: Breakup by Country (USD Billion), 2024-2032
20. Middle East and Africa In-Vitro Diagnostics Historical Market: Breakup by Country (USD Billion), 2018-2023
21. Middle East and Africa In-Vitro Diagnostics Market Forecast: Breakup by Country (USD Billion), 2024-2032
22. Global In-Vitro Diagnostics Market Structure
In 2023, the global in-vitro diagnostics market attained a value of nearly USD 71.90 billion.
The market is projected to grow at a CAGR of 4.5% between 2024 and 2032.
The market is estimated to witness a healthy growth in the forecast period of 2024-2032 to reach USD 106.79 billion by 2032.
The major drivers of the market include rising population, growing urbanisation, increasing demand for advanced diagnostic facilities and shift towards rapid testing.
Technological advancements and the increasing focus on safety and accuracy are the key industry trends propelling the growth of the market.
The major regions in the industry are North America, Latin America, the Middle East and Africa, Europe, and the Asia Pacific.
The product and service types include reagents and kits, instruments, and software and services.
The technological segments are immunodiagnostics, clinical chemistry, molecular diagnostics, haematology, microbiology, coagulation and haemostasis, and urinalysis, among others.
The diverse applications for market are infectious diseases, diabetes, oncology, cardiology, drug testing/pharmacogenomics, HIV/AIDS, autoimmune diseases, and nephrology, among others.
The end users for the industry consist of hospital and clinics, diagnostic laboratories, point-of-care testing, and academic institutes, among others.
The major players in the industry are Danaher Corp, bioMérieux, Inc., Siemens Healthcare GmbH, ARKRAY America, Inc., Sysmex Corporation, and F. Hoffmann-La Roche Ltd, among others.
The global in-vitro diagnostics market attained a value of USD 71.90 billion in 2023, driven by the rising rapid testing. Aided by the growing technological advancements, the market is expected to witness a further growth in the forecast period of 2024-2032, growing at a CAGR of 4.5%. The market is projected to reach USD 106.79 billion by 2032.
EMR’s meticulous research methodology delves deep into the market, covering the macro and micro aspects of the industry. Based on its product and service types, the in-vitro diagnostics industry can be segmented into reagents and kits, instruments, and software and services. The classification based on technology are immunodiagnostics, clinical chemistry, molecular diagnostics, haematology, microbiology, coagulation, and haemostasis, and urinalysis, among others. On the basis of applications, the industry is divided into infectious diseases, diabetes, oncology, cardiology, drug testing/pharmacogenomics, HIV/AIDS, autoimmune diseases, and nephrology, among others. The end uses for the product include hospital and clinics, diagnostic laboratories, point-of-care testing, and academic institutes, among others. The major regional markets for in-vitro diagnostics are North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa. The key players in the above market include Danaher Corp, bioMérieux, Inc., Siemens Healthcare GmbH, ARKRAY America, Inc., Sysmex Corporation, and F. Hoffmann-La Roche Ltd, among others.
EMR’s research methodology uses a combination of cutting-edge analytical tools and the expertise of their highly accomplished team, thus, providing their customers with market insights that are accurate, actionable, and help them remain ahead of their competition.
Mini Report
-
Selected Sections, One User
-
Printing Not Allowed
-
Email Delivery in PDF
-
Free Limited Customisation -
Post Sales Analyst Support -
50% Discount on Next Update
Single User License
-
All Sections, One User
-
One Print Allowed
-
Email Delivery in PDF
-
Free Limited Customisation -
Post Sales Analyst Support -
50% Discount on Next Update

Five User License
-
All Sections, Five Users
-
Five Prints Allowed
-
Email Delivery in PDF
-
Free Limited Customisation
-
Post Sales Analyst Support
-
50% Discount on Next Update
Corporate License
-
All Sections, Unlimited Users
-
Unlimited Prints Allowed
-
Email Delivery in PDF + Excel
-
Free Limited Customisation
-
Post Sales Analyst Support
-
50% Discount on Next Update
Any Question? Speak With An Analyst
View A Sample
Did You Miss Anything, Ask Now
Right People
We are technically excellent, strategic, practical, experienced and efficient; our analysts are hand-picked based on having the right attributes to work successfully and execute projects based on your expectations.
Right Methodology
We leverage our cutting-edge technology, our access to trusted databases, and our knowledge of the current models used in the market to deliver you research solutions that are tailored to your needs and put you ahead of the curve.
Right Price
We deliver in-depth and superior quality research in prices that are reasonable, unmatchable, and shows our understanding of your resource structure. We, additionally, offer attractive discounts on our upcoming reports.
Right Support
Our team of expert analysts are at your beck and call to deliver you optimum results that are customised to meet your precise needs within the specified timeframe and help you form a better understanding of the industry.