From Theory to Real-World Practice: Clinical Integration of Digital Therapeutics
Digital therapeutics may be the biggest paradigm-shifting change medicine has seen yet...
metamorworks/AdobeStock

Digital therapeutics (DTx) is a new category of medicine that refers to treatments delivered directly to patients via software or apps. The scope of DTx encompasses treatment, management, and prevention of a broad spectrum of diseases and disorders. As such, DTx are part of a larger ecosystem of digital health technologies that provides support for patients, clinicians, and medical institutions. These technologies include patient- and clinician-platforms, as well as the unseen operational components.
DTx differs from the multitude of mental health apps on the market because they are meant to be prescribed or recommended by a health care professional; part of a comprehensive treatment plan; evidence-based; and subject to the same regulatory requirements as traditional medical treatments. The development of DTx is an area that affects patients, clinicians, payors, and policymakers. The Digital Health Alliance1 has put forth a 10-point list of core principles for DTx products as a first step in operationalizing the industry; these principles also help to distinguish DTx products from unregulated apps.
In order to use DTx effectively, we must understand its relationship to the following categories: patients, clinicians, health care systems, and innovation/discovery, especially as it relates to comorbid disorders.
DTx and Patients
As many as 80% of adults in the US have a smartphone, and many of them spend hours per day interacting with their devices.2 Although most of that time is likely for entertainment purposes, there are also opportunities to improve their health.
Recent advances in technology have led to the unobtrusive, seamless collection of patient-generated health data from smartphones, which can be used to improve patient care and outcomes. For example, smartphones can collect data on sleep, activity, physiology, and device use, as well as provide an environmental context. Digital phenotyping refers to the “moment-by-moment quantification of the individual-level human phenotype in situ, using data from smartphones and other personal devices,” and DTx takes it to the next level by recommending actions based on that phenotyping.1
The near ubiquity of these devices means that the clinical applications of digital phenotyping and DTx are highly scalable; their use also allows the individuals’ data to be used as their own unique baseline. Research has demonstrated the feasibility and acceptability of physiological data capture for various disorders.3 The hope is that digital markers of behavioral change (ie, observable changes in sleep, physical activity, and social interaction) might become sensitive measures of meaningful variation in functional status, symptoms, and risk for adverse outcomes in patients, thereby better guiding and personalizing care.3,4
Acceptability of app use, and DTx in general, is an encouraging sign. Scalability will likely depend on age and generational influences (younger individuals may potentially be more amenable to DTx use), comfort with technology in general, and socioeconomic disparities (ie, more affluent individuals with better phones/wearables will be able to access more nuanced DTx options).
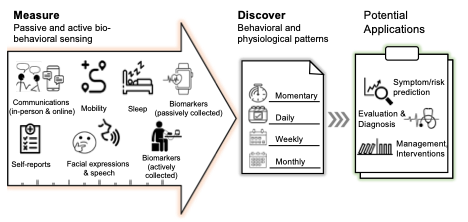
A yet unexplored problem is adherence to DTx use once prescribed; as large-scale implementation becomes a reality, this issue will need to be appropriately addressed. “App burnout,” a phenomenon referring to the short-term use of apps, may be relevant to DTx as the prescribed length of time (ie, weeks, months, years) increases. Current studies in mental health, for instance, have looked at DTx with contingency management and cognitive behavioral therapy for 12 to 16 weeks; medication active sensing systems meant to improve adherence, such as Abilify MyCite (aripiprazole tablets with sensor), have been helpful in short-term studies and have been evaluated for a maximum of 26 weeks per the United States Food and Drug Administration (FDA) approval. Thus, although the short-term implementation of DTx has been an attractive option for the management of chronic disorders, much is still unknown about the interaction of DTx with the naturalistic course of a chronic illness, and much remains unknown about the best times to implement DTX measures (Figure).
Figure. Extracting Biobehavioral Markers From Sensors for the Development of DTX
Clinician Perspectives
DTx have been developed over the past 15 to 20 years to target chronic medical and mental issues, with the assumption that many of these conditions can be improved by shaping behaviors. However, physicians adopting DTx in chronic management face ethical, legal, and practical challenges. In the case of psychotic disorders, ingestible sensors (despite positive data in promoting adherence) are seen as potentially decompensating factors in a patient’s delusion, thus limiting adoptability.5,6
Although not strictly DTx, deep brain stimulation devices have faced adoptability challenges for depressive and psychotic disorders because of clinician concerns that they may worsen the primary disease.7 Gartner Inc described the concept of the hype cycle, a maturity technology timeline that relates technology visibility to time. This concept explains the issues involved in DTx adoptability, including novelty of the new modality, expected value, expectation inflation of opinion related to innovation, and time needed for the technology itself to reach maturity.8
Clinicians theoretically support the idea of closing treatment gaps and would likely be open to leveraging concrete treatment planning and adherence tools to reinforce what was discussed in-session. However, despite FDA approval of DTx apps (such as Welldoc’s BlueStar for Type II diabetes management and Pear Therapeutics’ reSET for the treatment of substance use disorder), DTx is still globally an underprescribed and underused modality. In addition, the adoption of DTx in clinical practice has been slow.9 Why is this the case?
From a clinician perspective, there are a number of hurdles to overcome, including lack of familiarity with DTx, lack of time to introduce and administer DTx, and the lack of DTx integration with electronic health records. Similarly, there are obstacles in billing for DTx. Although they exist, very few physicians are employed in systems that offer digital formularies. Thus, adoption by physicians is an unsolved challenge for DTx implementation. In order to achieve DTx scalability, it is necessary to change the clinical visit microenvironment and make DTx a viable treatment decision.
Systemic and Regulatory Perspectives
Reimbursement and oversight are the 2 big areas of concern in health care systems, yet the regulatory landscape surrounding DTx is still in flux.10 The first question that must be answered is who is responsible for regulating DTx. The second is, what is the relation of DTx to machine learning and whether changes in software or updated machine learning algorithms would require reapproval or new approvals. The Total Product Lifecycle approach currently under consideration by the FDA could address some of these concerns.
Currently, approved or cleared products have received their acceptance from the FDA Center for Devices and Radiological Health, some using the Breakthrough Therapy designation. The aim, ideally, would be a path for software-as-a-medical-device (SaMD) products, with predetermined steps through the lifecycle of the software. This will require modernization of the FDA paths for medical devices, which were written in 1976.10
System-level challenges of DTx also include cybersecurity. DTx interfaces with, and is reliant on, multiple nonmedical entities, including internet, phone, and cloud storage service providers. There are currently no global answers to these issues, but patients and clinicians alike will be reluctant to transmit sensitive health data over unsecured channels. Moreover, reimbursement for DTx is moving slowly but surely. Some private insurers pay for DTx as prescribed, but nationwide reimbursement codes remain uncommon.
The Power of Innovation
Thus far, the main DTx developments have been the delivery of already proven treatments via electronic/software means. However, the future of DTx will likely include higher-order constructs and products that address comorbid disorders. Digital innovation in mental health already exists, and is accelerating rapidly, which is a good thing. Digital innovation serves 3 broad purposes.
First, digital innovation can increase access to mental health care. With the currently available technologies, there has been a continual improvement in the delivery of mental health services via telemedicine/telepsychiatry. Telepsychiatry has been important in facilitating patient follow-up by reducing travel, thereby helping longitudinal engagement. This, in turn, has improved adherence and reduces loneliness and misinformation.11 Although telepsychiatry may not always be optimal for patient engagement and patient-physician rapport, studies have shown it is not inferior to face-to-face care.11 Feasibility and success in delivering therapeutic interventions, such as cognitive behavioral or supportive therapy, and sleep hygiene education via the internet/phone/apps have been demonstrated.12-14
Second, digital innovations provide a new arsenal of tools to measure aspects of patient biology and behavior in ways that were not previously possible. This objective data collection, in turn, will likely allow better diagnosis and will allow clinicians to adjust care, as necessary.
Third, the fast-expanding repertoire of artificial intelligence tools enables the discovery of complex relationships between new forms of data and mental health conditions. As a result, novel ideas are emerging to quantify complex concepts like loneliness, mood swings, and cravings/binge eating that may underlie worsening mental health symptoms. Future DTx developments are expected to go beyond already proven treatments.
Mobile and Wearable Technologies in Mental Health
Seppälä et al15 reviewed mobile phone/wearable sensor–based mobile studies (N = 33) for mental health conditions between 2009 and 2018. The majority of the studies targeted unipolar depression or anxiety in healthy participants, with fewer studies focusing on bipolar and psychotic disorders. The Global Positioning System (GPS) was the most common sensor used. Features such as reduced mobility patterns and time spent at home during specific time intervals of the day were associated with scores indicative of depression or anxiety, as measured by questionnaires such as the Patient Health Questionnaire (PHQ)-9, Generalized Anxiety Disorder (GAD)-7, or by Ecological Momentary Assessment (EMA) self-reported mood.15,16 The second most common assessment was physical activity, measured with an accelerometer/gyroscope; this was followed by phone call logs.16
Higher-level clinically relevant constructs, such as behavioral regularity, may be useful to better understand behavior patterns. Data from wearable and mobile phone sensors showed that 2 behavioral regularity indices17 were correlated with perceived stress scale scores, the mental component scale of the 12-Item Short Form Health Survey (SF-12), and daily self-reported mood.17 The indices included a sleep regularity index that quantified the regularity of sleep time, wake time, and sleep duration, and a daily mobility index that detailed routines based on GPS data. Using behavioral rhythm markers (eg, ultradian, circadian, and infradian rhythms) estimated from mobile phone sensors, these data predicted self-reported symptoms from individuals with schizophrenia (the machine learning algorithm is able to predict when patients will be reporting certain symptoms based on behavioral patterns collected as above).18,19 Changes in daily behavioral patterns based on mobile phone sensors were used to predict symptom resurgence in patients with schizophrenia.19
The higher-level construct of social ambiance can likewise be obtained by unconstrained day-long recordings from wrist-worn audio-bands that estimate the number of simultaneous speakers.20 The number of simultaneous speakers was used as a proxy for an individual’s sociability, as social isolation is often a symptom of mental illness. By classifying audio into 4 levels (ie, quiet, low, mid, and high social ambiance), researchers found that individuals with depression or psychosis spent less time in diverse environments with higher social ambiance levels. Moreover, social ambiance patterns are associated with the severity of self-reported depression and anxiety symptoms, and personality traits such as neuroticism and agreeableness.
Despite strong preliminary evidence associating sensor data with mental health conditions, many challenges lie ahead in data-driven modeling and inference. This issue is a major challenge associated with the scalability of DTx development, as it requires industry and academic collaborations. For instance, computational models need to learn associations between individual patients’ biomarkers and mental health constructs (eg, symptoms, critical events). Every patient has different behavioral patterns and physiological responses, so the challenge becomes, how to determine regular/irregular patterns for each patient, or each group of patients.
A single computational model might not be able to fit the heterogeneity of different patients. Similarly, patients’ symptoms are based on their self-reports, which are subject to variabilities and biased perceptions. Current technology has a limited ability to detect biased self-reports, thereby limiting the usability of deep learning. Deep learning is a popular and powerful approach to developing complex neural network models; however, it is hard to interpret why certain models work well, and model-building requires a significant amount of data per patient.
Comorbid Disorders and Paradigm Shifts
Although the overlap between depressive disorders and diabetes is not well understood, it has been hypothesized to be potentially linked to immune system overactivity/systemic inflammation, psychoneuroendocrine dysregulation, cognitive functioning, and common genetic risk factors.4,11,21,22 This type of comorbidity could be effectively targeted with DTx if models are built to understand this disease overlap,23 especially as the field undergoes a paradigm shift.
One of the biggest conceptual ongoing shifts in psychiatry is characterizing mental illnesses dimensionally, in contrast to the current practice of categorical classification. Taking major depressive disorder (MDD) as an example, a shift in both diagnosis and management can be achieved by leveraging technology and by adopting a more modern, innovative approach to diagnosis. In the current diagnostic paradigm, MDD is defined in the DSM-5 as having 5 of 9 symptoms (with necessary inclusion of depressed mood and/or anhedonia). Thus, it is possible that 2 patients with the same MDD diagnosis have no overlapping symptoms: one has depressed mood, the other anhedonia; one eats too much, one eats too little; one sleeps too much, the other too little; one is suicidal, the other is not, etc.
The National Institute of Mental Health has established the Research Domain Criteria (RDoC) program, which recommends that mental health research be focused on symptoms instead of diagnoses, and studies should be done not categorically, but dimensionally, in a manner that allows overlap with tech-based objective information gathering. The RDoC highlights the following neurobiological transdiagnostic research dimensions: negative valence, positive valence, cognitive processes, systems for social processes, and arousal/regulatory systems. The current state of DTx is patient-centered/patient-driven information collection, but most DTx structures, thus far, do not fit categorical or dimensional psychiatric diagnostic frameworks; instead, they focus on individual symptoms and may be the simplest level of dimensional classification.
In the dimensional paradigm, a symptom like low energy can be translated into low physical activity/low mobility/low socialization by sensor detection. Those, in turn, can be addressed with a focused intervention, such as behavioral activation. This approach has measurable outcomes, is easy to explain to patients, and is more amenable to process improvement based on real-time feedback. The current approach to MDD treatment, which usually starts with the addition of a selective serotonin reuptake inhibitor (SSRI), aims to improve feelings of depression/anhedonia generating the low activity/low energy.
Going back to the issue of comorbidity, taking an SSRI provides some improvement in survival in the context of diabetes, but not the expected protective effect that is hoped for in diabetes and depression.24,25 Intuitively, this makes sense as a lifting mood may not fully translate into the desired energy increase, unless the patient specifically learns the needed skills to effect this change.
The field has turned to dimensions that may impact behavioral change, and those in turn will need to be measured and relayed back to the patient in a feedback loop. A good illustration of this is self-efficacy, or the belief that one has control over health behaviors. Self-efficacy tends to be diminished in patients with chronic illnesses, who may accept many symptoms as an inevitable part of the disease process, and lack the understanding/ability to take control of their biological processes. Poor self-efficacy may overlap with the social determinants of health and health care disparities but, in general, studies on self-efficacy and specific diabetes-related mitigating behaviors are promising.26-28 Taking care of oneself would help to improve mobility/energy and, by extension, mood. At this point, this stepped process lacks clarity and established protocols, but it could be an excellent DTx goal for further development.
Concluding Thoughts
Leveraging DTx to improve mental and physical health is likely to be the biggest, paradigm-shifting change that medicine has known since the invention of antibiotics. However, how to apply these tools remains an area that needs operationalization. DTx is at the nexus of digital innovation and scalability/wide-scale use of digital interventions, with more exciting developments on the horizon.
Dr Moukaddam is associate professor, Menninger Department of Psychiatry & Behavioral Sciences, Baylor College of Medicine, and Ben Taub Adult Outpatient Services Director, Medical Director, Stabilization, Treatment & Rehabilitation (STAR) Program for Psychosis. Dr Sano is an Assistant Professor at Rice University, Department of Electrical and Computer Engineering, Computer Science, and Bioengineering. She also directs the Computational Wellbeing Group. Dr Salas is an associate professor of Psychiatry Research at Baylor College of Medicine. Dr Sabharwal is chair of the Department of Electrical and Computer Engineering and the Earnest Dell Butcher professor of engineering at Rice University.
References
1. Digital Therapeutics Alliance. Transforming Global Healthcare by Advancing Digital Therapeutics. Accessed June 14, 2021. https://dtxalliance.org
2. Statista. How much time on average do you spend on your phone on a daily basis? Accessed June 22, 2021. https://www.statista.com/statistics/1224510/time-spent-per-day-on-smartphone-us/
3. Brannon EE, Cushing CC, Crick CJ, Mitchell TB. The promise of wearable sensors and ecological momentary assessment measures for dynamical systems modeling in adolescents: a feasibility and acceptability study. Transl Behav Med. 2016;6(4):558-565.
4. Kazukauskiene N, Podlipskyte A, Varoneckas G, Mickuviene N. Insulin resistance in association with thyroid function, psychoemotional state, and cardiovascular risk factors. Int J Environ Res Public Health. 2021;18(7):3388.
5. Gerke S, Babic B, Evgeniou T, Cohen IG. The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. NPJ Digit Med. 2020;3:53.
6. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia. Clin Schizophr Relat Psychoses. Published online January 3, 2019.
7. Gardner J. A history of deep brain stimulation: technological innovation and the role of clinical assessment tools. Soc Stud Sci. 2013;43(5):707-728.
8. Dedehayir O, Steinert M. The hype cycle model: a review and future directions. Technol Forecast Soc Change. 2016;108:28-41.
9. Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. NPJ Digit Med. 2020;3(1):14.
10. Patel NA, Butte AJ. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit Med. 2020;3(1):159.
11. Batastini AB, Paprzycki P, Jones ACT, MacLean N. Are videoconferenced mental and behavioral health services just as good as in-person? A meta-analysis of a fast-growing practice. Clin Psychol Rev. 2021;83:101944.
12. Barnes DM, Jarlais DD. Feasibility of a simple and scalable cognitive-behavioral intervention to treat problem substance use. J Subst Use. 2019;24(6):693-695.
13. Erten Uyumaz B, Feijs L, Hu J. A review of digital cognitive behavioral therapy for insomnia (CBT-I Apps): are they designed for engagement? Int J Environ Res Public Health. 2021;18(6):2929.
14. Koppel R, Kuziemsky C. Usability across health information technology systems: searching for commonalities and consistency. Stud Health Technol Inform. 2019;264:649-653.
15. Seppälä J, De Vita I, Jämsä T, et al. Mobile phone and wearable sensor-based mHealth approaches for psychiatric disorders and symptoms: systematic review. JMIR Ment Health. 2019;6(2):e9819.
16. Boonstra TW, Nicholas J, Wong QJ, et al. Using mobile phone sensor technology for mental health research: integrated analysis to identify hidden challenges and potential solutions. J Med Internet Res. 2018;20(7):e10131.
17. Saeb S, Zhang M, Karr CJ, et al. Mobile phone sensor correlates of depressive symptom severity in daily-life behavior: an exploratory study. J Med Internet Res. 2015;17(7):e175.
18. Lamichhane B, Ben-Zeev D, Campbell A, et al. Patient-independent schizophrenia relapse prediction using mobile sensor based daily behavioral rhythm changes. Paper presented at: Wireless Mobile Communication and Healthcare: 9th EAI International Conference, MobiHealth 2020, Virtual Event; November 19, 2020. Proceedings 92021.
19. Tseng VW, Sano A, Ben-Zeev D, et al. Using behavioral rhythms and multi-task learning to predict fine-grained symptoms of schizophrenia. Sci Rep. 2020;10(1):15100.
20. Chen W. AmbianceCount: an objective social ambiance measure from unconstrained day-long audio recordings. Master’s thesis. Rice University; 2020.
21. Repple J, König A, de Lange SC, et al. Association between genetic risk for type 2 diabetes and structural brain connectivity in major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;S2451-9022(21)00056-2.
22. Reus GZ, Carlessi AS, Silva RH, et al. Relationship of oxidative stress as a link between diabetes mellitus and major depressive disorder. Oxid Med Cell Longev. 2019;2019:8637970.
23. Sabharwal A, Fields SA, Hilliard ME, DeSalvo DJ. Digital technologies to support behavior change: challenges and opportunities. In: Diabetes Digital Health. 2020:37-50.
24. Avrahamy H, Shoval G, Hoshen M, et al. Association between adherence to SSRI treatment and mortality among individuals with metabolic syndrome components. Pharmacopsychiatry. Published online April 14, 2021.
25. Lee HM, Yang YC, Chen SF, et al. Risk of hyperglycemic crisis episode in diabetic patients with depression: a nationwide population-based cohort study. J Diabetes Complications. 2020;34(3):107509.
26. Brown KK, Kindratt TB, Boateng GO, Brannon GE. Racial and ethnic disparities in healthcare rating, diabetes self-efficacy, and diabetes management among non-pregnant women of childbearing age: does socioeconomic status matter? J Racial Ethn Health Disparities. Published online April 7, 2021.
27. McElfish PA, Rowland B, Scott AJ, et al. Examining the relationship between physical activity and self-efficacy for exercise among overweight and obese Marshallese adults. J Immigr Minor Health. Published online April 10, 2021.
28. Pereira HV, Palmeira AL, Encantado J, et al. Systematic review of psychological and behavioral correlates of recreational running. Front Psychol. 2021;12:624783.
