Reports
Sale

Global Gene Therapy Market Size, Share, Growth, Trends: By Therapy Type: In-Vivo Therapy, Ex-Vivo Therapy; By Indications: Acute Lymphoblastic Leukemia (ALL), Inherited Retinal Disease, Large B-Cell Lymphoma, ADA-SCID, Melanoma, Beta-Thalassemia Major/SCD, Others; By Vector Type; Regional Analysis; Supplier Landscape; 2024-2032
Global Gene Therapy Market Outlook
The gene therapy market size attained a value of USD 7.81 billion in 2023. The market is anticipated to grow at a CAGR of 22.8% during the forecast period of 2024-2032 to attain a value of USD 49.60 billion by 2032. The growth of the market is driven by increased funding and investments.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
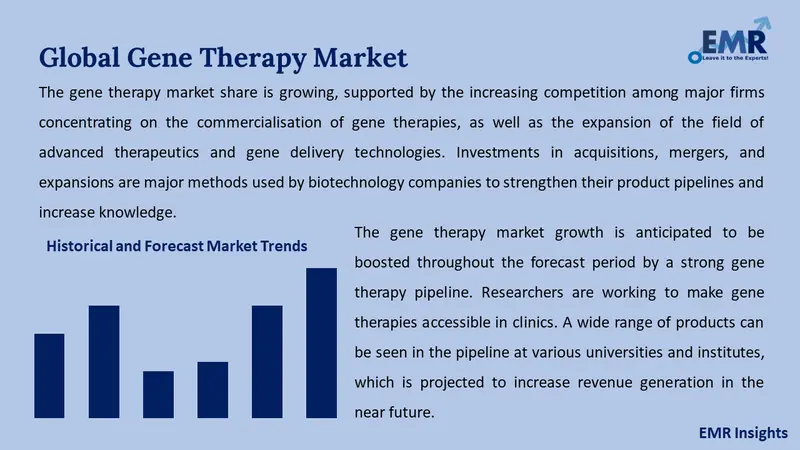
The gene therapy market share is growing, supported by the increasing competition among major firms concentrating on the commercialisation of gene therapies, as well as the expansion of the field of advanced therapeutics and gene delivery technologies. Investments in acquisitions, mergers, and expansions are major methods used by biotechnology companies to strengthen their product pipelines and increase knowledge.
Also, increased funding and investments are anticipated to open up profitable growth potential for market participants in gene therapy. Recent years have seen a significant increase in the gene therapy market mainly due to the rapid technological advances in cellular and molecular biology and genomics research.
The gene therapy market growth is anticipated to be boosted throughout the forecast period by a strong gene therapy pipeline. Researchers are working to make gene therapies accessible in clinics. A wide range of products can be seen in the pipeline at various universities and institutes, which is projected to increase revenue generation in the near future.
Increasing Applications of Viral Vectors in Gene Therapy for the Treatment of Chronic Diseases Driving the Market Growth
The increasing applications of viral vectors in gene therapy for the treatment of neurological, metabolic, and neurodegeneration diseases are driving the segment growth. According to the gene therapy market report, viral vectors offer various favourable properties such as safety, low-toxicity, and excellent stability, due to which they are increasingly preferred in gene therapy. As adenoviruses have excellent DNA harbouring capacity, they are one of the most extensively applied viral vectors in gene therapy. They are also used for the development of vaccines and cancer therapies, hence fuelling the growth of the gene therapy market.
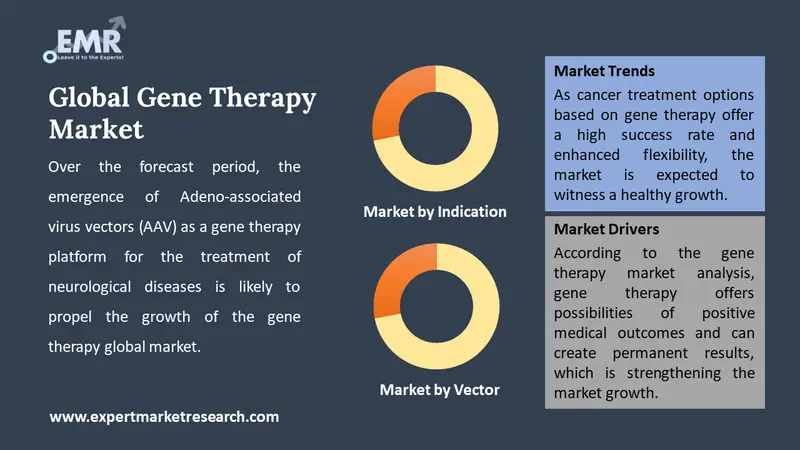
As per the gene therapy market forecast, viral vectors account for a significant share in the clinical trials of gene therapy owing to their efficiency to deliver genes to the target tissues/cells. The increasing regulatory approvals of gene therapy drugs based on viral vectors are further propelling the market. Over the forecast period, the emergence of Adeno-associated virus vectors (AAV) as a gene therapy platform for the treatment of neurological diseases is likely to propel the growth of the gene therapy global market.
North America Accounts for a Significant Share in the Market for Gene Therapy
The gene therapy market in North America is being driven by the growing healthcare expenditure in the region and a strong foothold of major biopharmaceutical companies focused on advancing gene therapy techniques. According to the gene therapy market research, the growing prevalence of health issues such as cancer and cystic fibrosis in the region is further propelling the demand for gene therapy. In addition, the surging initiation of gene therapy clinical trials in the region to develop effective treatments for chronic diseases is augmenting the market. Moreover, the introduction of various favourable initiatives by various governments to encourage innovations and advancements in gene therapy is likely to propel the market expansion. The surging approval rates of new gene therapies by regulatory authorities to expand therapeutic treatment choices are expected to further aid the cell and gene therapy market growth in the forecast period.
Market Segmentation
Gene therapy is defined as a medical approach that targets the genetic problem of a patient in order to treat or prevent diseases. In this treatment therapy, a person’s genetic make-up is altered or modified instead of using drugs or surgery. Several mechanisms, including the replacement of disease-causing genes, inactivation of a disease-causing gene, and introduction of a modified gene into the body, among others, are involved in this process.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Based on indication, the market can be segmented into:
- Neurological Disorder
- Oncological Disorders
- Rare Diseases
- Eye Disorders
- Others
By vector, the market is divided into:
- Non-Viral Vector
- Oligonucleotides
- Others
- Viral Vector
- Retroviral Vectors
- Adeno-associated Viral Vectors
- Others
The EMR report looks into the regional markets of gene therapy like North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Emergence of Gene Therapy as a Promising Treatment Option for Various Diseases to Boost the Market Growth
The emergence of gene therapy techniques as a promising treatment option for various genetic diseases such as muscular dystrophy and cystic fibrosis, among others, is increasing the gene therapy market size. The emerging treatment modalities, such as immunotherapy and oncolytic virotherapy, are increasingly gaining traction in cancer treatments. In addition to this, the increasing prevalence of cancer, coupled with the robust research and development activities aimed at expanding cancer treatment options, is likely to accelerate the growth of the cancer gene therapy market in the coming years. As cancer treatment options based on gene therapy offer a high success rate and enhanced flexibility, the market is expected to witness a healthy growth.
According to the gene therapy market analysis, gene therapy offers possibilities of positive medical outcomes and can create permanent results, which is strengthening the market growth. Over the forecast period, the increasing approvals of various gene therapy medications by regulatory authorities like the Food and Drug Administration (FDA) of the United States are expected to bolster the gene therapies market growth. Moreover, the evolving patient preference toward minimally invasive treatment procedures for diseases like lymphoma and leukaemia, among others, is anticipated to propel the gene therapy market growth in the coming years.
Key Players in the Global Market for Gene Therapy
The report gives a detailed analysis of the following key players in the global gene therapy market, covering their competitive landscape, capacity, and latest developments like mergers, acquisitions, and investments, expansions of capacity, and plant turnarounds:
- Novartis AG
- Amgen Inc.
- bluebird bio, Inc.
- Biogen Inc.
- Sibiono GeneTech Co. Ltd.
- Gilead Sciences, Inc.
- Sangamo Therapeutics, Inc.
- Intellia Therapeutics, Inc.
- REGENXBIO Inc.
- Abeona Therapeutics Inc.
- Orchard Therapeutics plc
- Sio Gene Therapies Ltd.
- Ultragenyx Pharmaceutical Inc.
- Krystal Biotech, Inc.
- Rocket Pharmaceuticals, Ltd.
- MeiraGTx Limited.
- Solid Biosciences Inc.
- GenSight Biologics S.A.
- Editas Medicine, Inc.
- CRISPR Therapeutics AG
- Spark Therapeutics, Inc.
- Genprex, Inc.
- Poseida Therapeutics, Inc.
- Astellas Pharma Inc.
- Voyager Therapeutics, Inc.
- Coave Therapeutics
- Precision BioSciences, Inc.
- Others
The comprehensive EMR report provides an in-depth assessment of the market based on the Porter's five forces model along with giving a SWOT analysis.
Key Highlights of the Report
| REPORT FEATURES | DETAILS |
| Base Year | 2023 |
| Historical Period | 2017-2023 |
| Forecast Period | 2024-2032 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Therapy Type |
|
| Breakup by Indications |
|
| Breakup by Vector Type |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Supplier Landscape |
|
| Companies Covered |
|
| Report Price and Purchase Option | Explore our purchase options that are best suited to your resources and industry needs. |
| Delivery Format | Delivered as an attached PDF and Excel through email, with an option of receiving an editable PPT, according to the purchase option. |
*At Expert Market Research, we strive to always give you current and accurate information. The numbers depicted in the description are indicative and may differ from the actual numbers in the final EMR report.
1 Preface
1.1 Objectives of the Study
1.2 Key Assumptions
1.3 Report Coverage – Key Segmentation and Scope
1.4 Research Methodology
2 Executive Summary
3 Global Gene Therapy Market Overview
3.1 Global Gene Therapy Market Historical Value (2017-2023)
3.2 Global Gene Therapy Market Forecast Value (2024-2032)
4 Global Gene Therapy Market Landscape
4.1 Global Gene Therapy Developers Landscape
4.1.1 Analysis by Year of Establishment
4.1.2 Analysis by Company Size
4.1.3 Analysis by Region
4.2 Global Gene Therapy Product Landscape
4.2.1 Analysis by Therapy Type
4.2.2 Analysis by Vector Type
4.2.3 Analysis by Indications
5 Global Gene Therapy Market Dynamics
5.1 Market Drivers and Constraints
5.2 SWOT Analysis
5.3 Porter’s Five Forces Model
5.4 Key Demand Indicators
5.5 Key Price Indicators
5.6 Industry Events, Initiatives, and Trends
5.7 Value Chain Analysis
6 Global Gene Therapy Market Segmentation
6.1 lobal Gene Therapy Market by Therapy Type
6.1.1 Market Overview
6.1.2 In-Vivo Therapy
6.1.3 Ex-Vivo Therapy
6.2 Global Gene Therapy Market by Indications
6.2.1 Market Overview
6.2.2 Acute Lymphoblastic Leukemia (ALL)
6.2.3 Inherited Retinal Disease
6.2.4 Large B-Cell Lymphoma
6.2.5 ADA-SCID
6.2.6 Melanoma
6.2.7 Beta-Thalassemia Major/SCD
6.2.8 Head & Neck Squamous Cell Carcinoma
6.2.9 Peripheral Arterial Disease
6.2.10 Spinal Muscular Atrophy (SMA)
6.2.11 Others
6.3 Global Gene Therapy Market by Vector Type
6.3.1 Market Overview
6.3.2 Viral Vector
6.3.2.1 Lentiviral Vectors
6.3.2.2 Adeno-Associated Viral (Aav) Vectors
6.3.2.3 Retrovirus Vectors
6.3.2.4 Modified Herpes Simplex Virus
6.3.2.5 Adenovirus Vectors
6.3.2.6 Others
6.3.3 Non-Viral Vector
6.4 Global Gene Therapy Market by Region
6.4.1 Market Overview
6.4.2 North America
6.4.3 Europe
6.4.4 Asia Pacific
6.4.5 Latin America
6.4.6 Middle East and Africa
7 North America Gene Therapy Market
7.1 Market Share by Country
7.2 United States of America
7.3 Canada
8 Europe Gene Therapy Market
8.1 Market Share by Country
8.2 United Kingdom
8.3 Germany
8.4 France
8.5 Italy
8.6 Others
9 Asia Pacific Gene Therapy Market
9.1 Market Share by Country
9.2 China
9.3 Japan
9.4 India
9.5 ASEAN
9.6 Australia
9.7 Others
10 Latin America Gene Therapy Market
10.1 Market Share by Country
10.2 Brazil
10.3 Argentina
10.4 Mexico
10.5 Others
11 Middle East and Africa Gene Therapy Market
11.1 Market Share by Country
11.2 Saudi Arabia
11.3 United Arab Emirates
11.4 Nigeria
11.5 South Africa
11.6 Others
12 Patent Analysis
12.1 Analysis by Type of Patent
12.2 Analysis by Publication year
12.3 Analysis by Issuing Authority
12.4 Analysis by Patent Age
12.5 Analysis by CPC Analysis
12.6 Analysis by Patent Valuation
12.7 Analysis by Key Players
13 Grants Analysis
13.1 Analysis by Year
13.2 Analysis by Amount Awarded
13.3 Analysis by Issuing Authority
13.4 Analysis by Grant Application
13.5 Analysis by Funding Institute
13.6 Analysis by NIH Departments
13.7 Analysis by Recipient Organization
14 Clinical Trials Analysis
14.1 Analysis by Trial Registration Year
14.2 Analysis by Trial Status
14.3 Analysis by Trial Phase
14.4 Analysis by Therapeutic Area
14.5 Analysis by Geography
15 Funding Analysis
15.1 Analysis by Type of Funding
15.2 Analysis by Funding Amount
15.3 Analysis by Leading Players
15.4 Analysis by Leading Investors
15.5 Analysis by Geography
16 Partnership and Collaborations Analysis
16.1 Analysis by Partnership Instances
16.2 Analysis by Type of Partnership
16.3 Analysis by Leading Players
16.4 Analysis by Geography
17 Regulatory Framework
17.1 Regulatory Overview
17.1.1 US FDA
17.1.2 EU EMA
17.1.3 INDIA CDSCO
17.1.4 JAPAN PMDA
17.1.5 Others
18 Supplier Landscape
18.1 AstraZeneca
18.1.1 Financial Analysis
18.1.2 Product Portfolio
18.1.3 Demographic Reach and Achievements
18.1.4 Mergers and Acquisitions
18.1.5 Certifications
18.2 Boehringer Ingelheim International GmbH
18.2.1 Financial Analysis
18.2.2 Product Portfolio
18.2.3 Demographic Reach and Achievements
18.2.4 Mergers and Acquisitions
18.2.5 Certifications
18.3 GlaxoSmithKline plc
18.3.1 Financial Analysis
18.3.2 Product Portfolio
18.3.3 Demographic Reach and Achievements
18.3.4 Mergers and Acquisitions
18.3.5 Certifications
18.4 Novartis AG
18.4.1 Financial Analysis
18.4.2 Product Portfolio
18.4.3 Demographic Reach and Achievements
18.4.4 Mergers and Acquisitions
18.4.5 Certifications
18.5 CHIESI Farmaceutici SpA
18.5.1 Financial Analysis
18.5.2 Product Portfolio
18.5.3 Demographic Reach and Achievements
18.5.4 Mergers and Acquisitions
18.5.5 Certifications
18.6 Sunovion Pharmaceuticals Inc
18.6.1 Financial Analysis
18.6.2 Product Portfolio
18.6.3 Demographic Reach and Achievements
18.6.4 Mergers and Acquisitions
18.6.5 Certifications
18.7 Teva Pharmaceutical Industries Ltd
18.7.1 Financial Analysis
18.7.2 Product Portfolio
18.7.3 Demographic Reach and Achievements
18.7.4 Mergers and Acquisitions
18.8 Mylan N.V., Orion Corporation
18.8.1 Financial Analysis
18.8.2 Product Portfolio
18.8.3 Demographic Reach and Achievements
18.8.4 Mergers and Acquisitions
18.8.5 Certifications
18.9 Merck & Co., Inc.
18.9.1 Financial Analysis
18.9.2 Product Portfolio
18.9.3 Demographic Reach and Achievements
18.9.4 Mergers and Acquisitions
18.9.5 Certifications
18.10 Grifols, S.A.
18.10.1 Financial Analysis
18.10.2 Product Portfolio
18.10.3 Demographic Reach and Achievements
18.10.4 Mergers and Acquisitions
18.10.5 Certifications
18.11 Abbott
18.11.1 Financial Analysis
18.11.2 Product Portfolio
18.11.3 Demographic Reach and Achievements
18.11.4 Mergers and Acquisitions
18.11.5 Certifications
18.12 F. Hoffmann-La Roche Ltd
18.12.1 Financial Analysis
18.12.2 Product Portfolio
18.12.3 Demographic Reach and Achievements
18.12.4 Mergers and Acquisitions
18.12.5 Certifications
18.13 Vectura Group plc
18.13.1 Financial Analysis
18.13.2 Product Portfolio
18.13.3 Demographic Reach and Achievements
18.13.4 Mergers and Acquisitions
18.13.5 Certifications
18.14 Pfizer Inc, Alkermes
18.14.1 Financial Analysis
18.14.2 Product Portfolio
18.14.3 Demographic Reach and Achievements
18.14.4 Mergers and Acquisitions
18.14.5 Certifications
18.15 Mylan N.V, Almirall, S.A, Genentech, Inc
18.15.1 Financial Analysis
18.15.2 Product Portfolio
18.15.3 Demographic Reach and Achievements
18.15.4 Mergers and Acquisitions
18.15.5 Certifications
18.16 Biogen
18.16.1 Financial Analysis
18.16.2 Product Portfolio
18.16.3 Demographic Reach and Achievements
18.16.4 Mergers and Acquisitions
18.16.5 Certifications
18.17 Astellas Pharma Inc.
18.17.1 Financial Analysis
18.17.2 Product Portfolio
18.17.3 Demographic Reach and Achievements
18.17.4 Mergers and Acquisitions
18.17.5 Certifications
19 Global Gene Therapy Market- Distribution Model (Additional Insight)
19.1 Overview
19.2 Potential Distributors
19.3 Key Parameters for Distribution Partner Assessment
20 Key Opinion Leaders (KOL) Insights (Additional Insight)
21 Company Competitiveness Analysis (Additional Insight)
21.1 Very Small Companies
21.2 Small Companies
21.3 Mid-Sized Companies
21.4 Large Companies
21.5 Very Large Companies
22 Payment Methods (Additional Insight)
22.1 Government Funded
22.2 Private Insurance
22.3 Out-of-Pocket
*Additional insights provided are customisable as per client requirements.
The global gene therapy market was valued at USD 7.81 billion in 2023.
The market is expected to grow at a CAGR of 22.8% from 2024 to 2032 to reach a value of USD 49.60 billion by 2032.
The major regions in the market are North America, Latin America, the Middle East and Africa, Europe, and the Asia Pacific.
The major drivers of the market include the increasing prevalence of chronic diseases, the growing approvals of gene therapy medications by health authorities, and the increasing inclination towards minimally invasive treatment procedures.
The growing research and development (R&D) activities by the major biopharmaceutical companies to expand gene therapy treatment options, the surging prevalence of cancer, and the growing initiation of gene therapy clinical trials.
Neurological disorder, oncological disorders, rare diseases, and eye disorders, among others, are the different indication segments of gene therapy.
Non-viral vector and viral vector are the significant vectors of gene therapy in the market.
The major players in the market are Novartis AG, Amgen Inc., bluebird bio, Inc., Biogen Inc., Sibiono GeneTech Co. Ltd., Gilead Sciences, Inc., Sangamo Therapeutics, Inc., Intellia Therapeutics, Inc., REGENXBIO Inc., Abeona Therapeutics Inc., Orchard Therapeutics plc, Sio Gene Therapies Ltd., Ultragenyx Pharmaceutical Inc., Krystal Biotech, Inc., Rocket Pharmaceuticals, Ltd., MeiraGTx Limited., Solid Biosciences Inc., GenSight Biologics S.A., Editas Medicine, Inc., CRISPR Therapeutics AG, Spark Therapeutics, Inc., Genprex, Inc., Poseida Therapeutics, Inc., Astellas Pharma Inc., Voyager Therapeutics, Inc., Coave Therapeutics, and Precision BioSciences, Inc., among others.
Mini Report
-
Selected Sections, One User
-
Printing Not Allowed
-
Email Delivery in PDF
-
Free Limited Customisation -
Post Sales Analyst Support -
50% Discount on Next Update
Single User License
-
All Sections, One User
-
One Print Allowed
-
Email Delivery in PDF
-
Free Limited Customisation -
Post Sales Analyst Support -
50% Discount on Next Update

Five User License
-
All Sections, Five Users
-
Five Prints Allowed
-
Email Delivery in PDF
-
Free Limited Customisation
-
Post Sales Analyst Support
-
50% Discount on Next Update
Corporate License
-
All Sections, Unlimited Users
-
Unlimited Prints Allowed
-
Email Delivery in PDF + Excel
-
Free Limited Customisation
-
Post Sales Analyst Support
-
50% Discount on Next Update
Any Question? Speak With An Analyst
View A Sample
Did You Miss Anything, Ask Now
Right People
We are technically excellent, strategic, practical, experienced and efficient; our analysts are hand-picked based on having the right attributes to work successfully and execute projects based on your expectations.
Right Methodology
We leverage our cutting-edge technology, our access to trusted databases, and our knowledge of the current models used in the market to deliver you research solutions that are tailored to your needs and put you ahead of the curve.
Right Price
We deliver in-depth and superior quality research in prices that are reasonable, unmatchable, and shows our understanding of your resource structure. We, additionally, offer attractive discounts on our upcoming reports.
Right Support
Our team of expert analysts are at your beck and call to deliver you optimum results that are customised to meet your precise needs within the specified timeframe and help you form a better understanding of the industry.