Impact of urease inhibitors on ammonia loss
Author: Helen Suter | Date: 06 Feb 2013
Helen Suter
Department of Agriculture and Food Systems, The University of Melbourne
GRDC project code: UM00037
Keywords: ammonia volatilisation, urea hydrolysis, urease inhibitor, NBPT
Take home messages
- Ammonia loss from surface applied granular urea can be high, with up to 30% loss recorded from pastures in southern Australia
- Ammonia loss from surface applied granular urea is highly dependent on micro-climatic conditions
- The majority of ammonia loss occurs within 4 to 10 days after fertiliser application depending on soil, crop and climatic conditions
- Urease inhibitors can reduce the peak of ammonia loss that occurs after fertiliser application
Introduction
Surface application of granular urea to crop and pasture systems can lead to large losses of ammonia (NH3) immediately following application and for about the next week to 10 days depending on the system. This is because the applied urea (CO(NH2)2) is hydrolysed to ammonium (NH4+) or ammonia gas (NH3) in a process that requires water and is catalysed by an enzyme ‘urease’ that is found in organic materials. The balance between NH4+ or NH3 is important and depends on pH, with a greater ratio of NH3 : NH4+ at higher pH (more alkaline). High pH can occur because of the soil environment or because of the localised pH hot spot that occurs around a urea granule as it hydrolyses. This localised hotspot drives NH3 loss.
Loss of N as NH3 is a concern only for surface applications of urea. Once the urea or NH3 are below the soil surface, either through irrigation or adequate rain washing it in, or through deep placement, the risk of N loss as NH3 is minimised. Urease inhibitors act by slowing the rate of urea hydrolysis (the process by which N is released from a granule), by inhibiting the action of the enzyme urease, and by doing this the elevated pH hotspot that occurs around the granule is lessened and this reduces the risk of NH3 loss. This then provides greater time for the urea to be washed into the soil. How well the inhibitor works depends on soil properties and climatic factors (temperature and moisture).
This paper presents laboratory and field experiment data that investigated the impact of the urease inhibitor NBPT (Green UreaTM 7 or 14 in Australia), on rates of urea and NH3 loss.
Laboratory experiments
In laboratory incubation experiments urea with and without the urease inhibitor NBPT (Green UreaTM) was applied to a range of different soil types under controlled environment conditions. These soils varied in pH, soil organic matter content and urease activity, which measures the rate at which urea is hydrolysed, and include: Western Victoria cropping soils, Mallee clayey sands, dairy pasture soils from south-western Victoria and north-central Victoria. Temperatures at which the experiments were conducted were designed to simulate possible surface soil temperatures that would exist at the time of fertiliser application, and ranged from 15 to 35oC. The temperature test was done to determine the effectiveness of the inhibitor, as it is known that the inhibitors work less well under higher temperatures.
Results for the south-western Victoria clay loam (pasture, pH 5.5, 20% clay, 3.9% organic C) and the Mallee clayey sand (cropping, pH 7.3, 9% clay, 0.5% organic C) are shown in Figure 1 and are typical responses for soils with high (a) and low (b) organic matter.
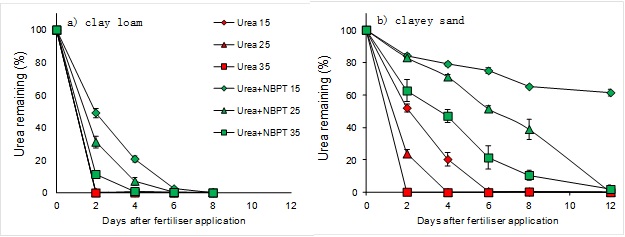
Figure 1. Urea remaining after fertiliser application with and without addition of the urease inhibitor NBPT in a clay loam and clayey sand under different incubation temperatures (15oC, 25oC, 35oC)
The typical responses we saw in these experiments were;
1) Urea hydrolysis was rapid in the more organic soils with urea completely hydrolysed within a couple of days. In the less organic soils the time for complete hydrolysis increased to between 6 and 8 days. Urea hydrolysis was more rapid at higher temperatures.
2) Using the urease inhibitor retained urea for longer. There was greater recovery of urea in the less organic soils than the organic soils. Increasing temperature reduced the impact of the inhibitor. Under cool temperature (15oC) around 60% of applied urea remained after 14 days in the less organic soils, whilst in the highly organic soils all urea was gone by 10 days.
[Relevant publication: Suter, H.C., Pengthamkeerati, P., Walker, C. and Chen, D.(2011) Influence of temperature and soil type on inhibition of urea hydrolysis by N-(n-butyl) thiophosphoric triamide in wheat and pasture soils in Southeastern Australia, Soil Research 49; 315-319.]
Field experiments
A field experiment was carried out on a ryegrass seed crop in south-western Victoria where surface applications of granular urea (40 kg N/ha) with and without a urease inhibitor were made in autumn and spring 2010. The experiment showed that NH3 loss in autumn was greater than in spring (Table 1) and that whilst the inhibitor was effective at both times, the real benefit in terms of N saved was only seen in autumn. The low rate of loss of NH3 in spring was due to the urea being applied to an already wet site and with rain falling within 24 hours of application. The wet site meant that urea was dissolved and therefore the elevated pH hotspot around the hydrolysing granules did not occur. In autumn however the site was drier but had a dew every morning which in combination with warming day temperatures and light wind, led to high losses as NH3. The majority of the NH3 lost from the urea occurred within the first 6 days after application.
Table 1. Ammonia lost from the ryegrass crop after surface application of granular urea.
| Autumn | Spring | |||
| Urea | Green Urea 14 | Urea | Green Urea 14 | |
| Ammonia loss (kg N/ha) | 12 | 3.6 | 0.8 | 0.5 |
| Ammonia loss (% of applied) | 30 | 10 | 2 | 1 |
| Value of lost N ($/ha)* | 7.8 | 2.7 | <1 | <1 |
* Assumes $650/t urea and $750 for Green Urea, transported and spread
Conclusions
Urease inhibitors can be used to reduce NH3 loss from surface applications of granular urea. The benefits achieved in terms of N saved, will depend upon the climate and in many cases use of a urease inhibitor coated onto the outside of a urea granule can be considered as a risk minimising strategy. The greatest benefit from the inhibitors will be in systems where high NH3 loss is expected (high soil organic matter and/or high soil pH systems, no/limited rainfall or irrigation, and some moisture; high wind speed near urea granules to move heavy NH3 gas away). Using urease inhibitors will enable farmers to reduce their N inputs as they may no longer need to allow for the N lost as NH3 at the time of fertiliser application, and this will reduce the quantity of fertiliser needed. This reduction in quantity needs to be balanced with the cost of the urease inhibitor product.
Contact details
Helen Suter
Department of Agriculture and Food Systems,
The University of Melbourne, Parkville, Vic 3010
03 8344 0179; 0438 456 602
helencs@unimelb.edu.au
GRDC Project Code: UM00037,
Was this page helpful?
YOUR FEEDBACK
